However it is important to pay special attention to the units used for density calculations.
Density computation for ceramics.
Mole tio2 86 58 mole zro2.
Melting points high 600 4000c thermal conductivities are low insulators thermal expansion values are low 1 15 ppm c 3.
Or by immersing the uncoated sample in a non.
This problem features the density formula for ceramics.
First you shall have to determine the bulk density using the formula.
Mass properties e g density ceramics are intermediate density 2 00 6 00 gms cm3 different for allotropes e g glass cristobalite tridymite quartz 2.
Lectures 22 and 23 introduction to ceramics ceramic density calculations example.
The theoretical density of the materials of the anode cathode and electrolyte are used to calculate the porosity of the materials a critical parameter in determining if the fuel cell will function properly.
Mass volume and then determine the theoretical density from xrd data using.
Calculate the density of feo given that it has the rock salt crystal structure.
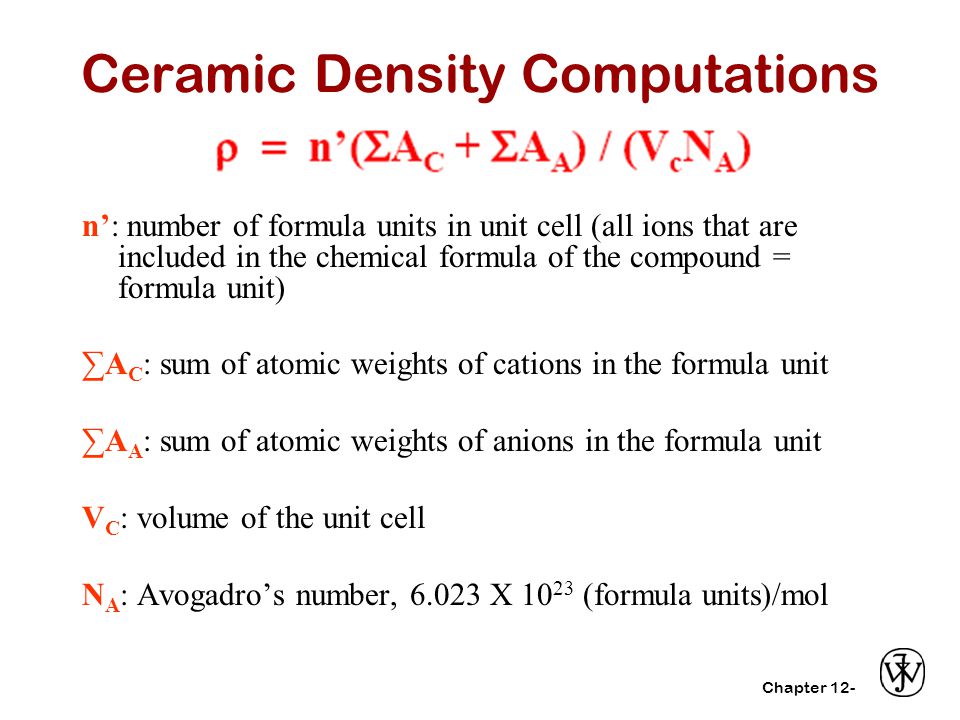
To compute for theoretical density of ceramics five essential parameters are needed and these parameters are number of formula units in unit cell n sum of atomic weights of atoms σa c sum of atomic weights of anions σa a unit cell volume v c and avogadro s number n a.
Mole sc2o3 0 92 mole hfo2 1 03 mole ceo2 1 4.
It is possible to access the bulk density of a porous ceramic sample by the archimedes method either by immersing a coated sample in a wetting liquid.
There are many different ways to express density and not using or converting into the proper units will result in an incorrect value.
Density calculations in ceramic structures c a c a v n n a a ρ n number of formula units in unit cell all ions that are included in the chemical formula of the compound formula unit σσσσac sum of atomic weights of cationsin the formula unit σσσσaa sum of atomic weights of anions in the formula unit vc volume of the unit cell.
The calculation of density is quite straightforward.
We use the equation c a fe o c a c a v n n a a v n n a a ρ since the crystal structure is rock salt n 4 formula units per unit cell and.